What We Do
KOTOBUKI Medical is a startup company based in Saitama, Japan. Using konjac, a gelatinous plant fiber, we create high-fidelity synthetic organs and surgical training systems. Our goal is to create a future in which surgery can be practiced safely without a heavy financial or environmental burden. We are actively connecting with healthcare professionals and the robotic surgical industry to improve surgical outcomes around the world.
Meet Our Team

CEO
Seiichiro TAKAYAMA

CSO
Hirotoshi UMEMOTO

CTO
Gaku MORIMOTO

Adviser
Koji NAKAO

Sales & Marketing Group
Nobuhiko UEHARA

Manufacturing and Development Group
Kiyotaka NAITO

Manufacturing and Development Group


Sales & Marketing Group
Heitaro KATSUNO

Manufacturing and Development Group
Ryosuke GOTO

Manufacturing and Development Group

Sales & Marketing Group


Manufacturing and Development Group
Kenta OZONO

Corporate Group
Mai KIKUYA

Sales & Marketing Groupp
Ayano IIZUKA

Manufacturing and Development Group
Yudai KOBAYASHI
Awards & Recognition
- 2015
- Winner of the 4th Shibusawa Eiichi Business Awards Special Prize for Venture Spirit
- 2015
- Good Design Award Winner (Laparo Training Binder)
- 2016
- Winner of the Saitama Prefecture Medical Device Prototype Contest Idea Award
- 2018
- Runner-up Grand Prix in the Saitama Prefecture Medical Device Prototype Contest
- 2018
- Grand Prize Winner of the Gunma Bank Business Support Award
History of the company
- 1978
- Shunju Takayama establishes KOTOBUKI Industries, a metalworking factory.
- 1984
- KOTOBUKI Giken, KOTOBUKI Medical’s parent company, is officially founded as a private corporation.
- 2005
- KOTOBUKI Giken is reorganized as a joint stock company with Seiichiro Takayama as the representative director.
- 2012
- Seiichiro Takayama begins developing and manufacturing surgical training products at KOTOBUKI Giken.
- 2014
- KOTOBUKI Giken is recognized as a Gold-Level Company for Diverse Work Practices.
- 2018
- KOTOBUKI Medical is established.
- 2019
- KOTOBUKI Medical breaks crowdfunding records at the stock investment site “FUNDINNO.”
- 2019
- KOTOBUKI Medical receives official funding from the Gunma Medical and Industrial Collaboration Activation Fund.
Message From the Founder
In Japanese, the kanji character for KOTOBUKI has several rich layers of meaning.
KOTOBUKI means a long life, but it also means celebration, abundance, and health. Japanese is a language
in which these many positive values can be elegantly conveyed with this one word, and that’s why we chose not to
translate the company’s name. KOTOBUKI is a word and a feeling that we wish to share with the world.
The kanji character for KOTOBUKI is also one part of my late father’s name. I want to share his legacy with the world by helping healthcare professionals and patients alike approach surgery with peace of mind.

Founder and CEO
With Stakeholders
The vision of KOTOBUKI Medical could never have been realizing without the generous support from our stakeholders.
To the many customers, local communities, joint researchers, investors, and more who continue to believe in us, we thank you.
KOTOBUKI Medical is committed to promoting sustainable business activities while strengthening our relationships with those who believed in us from the beginning.
Shareholders and Investors

We are forever grateful to the countless shareholders who invested in us through FUNDINNO, an investment-type crowdfunding sight operated by the Japan Crowd Capital. Through FUNDINNO’s platform, we are able to receive both encouragement and constructive criticism from our valued shareholders. We hope to continue to live up to your investment.
Our Customers

Our business is built on listening to the needs of the medical community. Some of our customers need a custom-made VTT organ with specific pathologies; others require a VTT model that can demonstrate the innovative power of their newly-launched medical device.
Whatever the need might be, we’re proud to have experience in working with customers not only in Japan, but also in Europe in the United States. We are careful to not only meet our clients’ needs, but also to faithfully follow through with their feedback and additional requests.
Manufacturing and craftsmanship are a part of KOTOBUKI Medical’s DNA. We are eager to press forward with expanding our client base and meeting every challenge and change in the medical industry head on.
Universities and Collaborators

We would never be able to develop quality products without the knowledge and expertise of medical professionals. To the physicians who have cooperated with our CEO Mr. Takayama since before KOTOBUKI Medical was established: we extend our most heartfelt, humble thanks. We will continue to value your trust and input, as well as the needs and feedback from the medical community as a whole.
OUR PROCESS
For 40 years, “KOTOBUKI” has been a name we are proud to associate with quality manufacturing. KOTOBUKI Medical’s parent company, Kotobuki Giken, has helped us cultivate decades of experience in metal processing, resin manufacturing, and the design of equipment and technology. Building on this legacy, KOTOBUKI Medical is dedicated to creating new tools that will forge a better future for the medical industry. It all begins with our process.
1.Development Based on Our
Clients’ Needs
Our process begins with the voices of our clients. We develop tools for medical and surgical training based on the needs of healthcare providers, medical suppliers, and education institutions. By starting our development with a listening ear, we ensure that our products have the best specifications for training and demonstration procedures.


2.Cost-Effective Prototyping
We make full use of KOTOBUKI Medical and KOTOBUKI Giken’s equipment and staff to create quality prototypes. One of our company’s root values is cost-efficiency. We guarantee training tools that maintain quality of build while considering the best cost performance.


3.Manufacturing Based on Feedback
When developing VTT models, KOTOBUKI Medical is dedicated to valuing and incorporating our clients’ feedback. We are always ready to adjust a model’s elasticity, design, and added morbidities to provide high-fidelity, quality simulations.
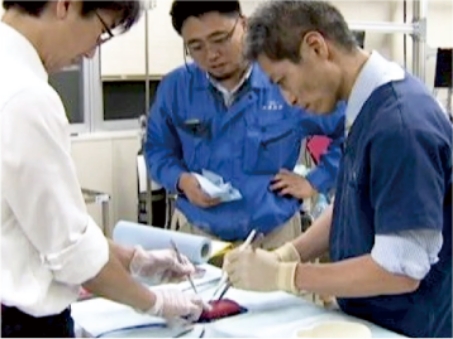
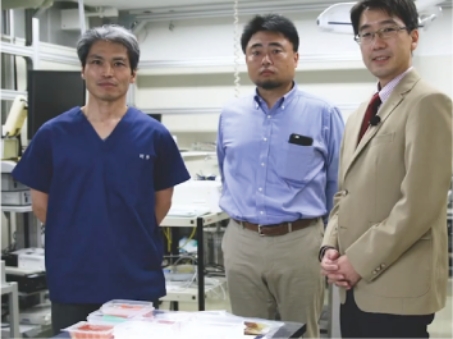
4.Research Beyond Manufacturing
We are constantly performing research to develop better products based on test results and feedback.
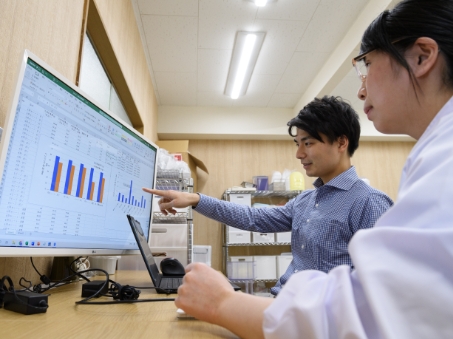

HEALTHCARE EQUITY FOR ALL
Introduction
Surgery is a consistently evolving field of medicine. As such, medical professionals are constantly having to adapt to new, minimally invasive technologies such as robotic surgery or endoscopic surgery.
With our surgical training kits and our high-fidelity synthetic tissue, KOTOBUKI Medical meets the needs of healthcare professionals who wish to improve their skills outside of the operating room. By supporting this vital part of the healthcare infrastructure, we aim to kickstart innovation in medical technology and help those in the industry give their patients the best quality of care.
Creating a Sustainable Future for Society, the Environment, and the Economy
We believe that to realize the society we are aiming for; it is essential to promote business activities and product development that consider not only economic aspects but also social and environmental aspects.
Society
KOTOBUKI Medical seeks to break down barriers to equipment installation and accessible training. By giving medical professionals a cost-effective, low-waste option for hands-on practice, we hope to relieve the ethical and economic burdens that can be created by surgical training methods.
Environment
Our VTT products are made of accessible plant-based ingredients that deliver less environmental impact than other plastic or silicon synthetic organs. This holds true for every step of the manufacturing process, from transportation and production to use and disposal.
Economy
Most high-fidelity human simulations cost thousands of dollars. Our models are a fraction of that price. We provide realistic training tools at a cost that is accessible to students and professionals alike.



★Sustainable Development Goals
By contributing to the improvement of technology for medical professionals through our products, we aim to help achieve "Goal 3: Good health and well-being" of the SDGs. The environmental and ethical impact of using VTT versus porcine or plastic prototypes can also contribute towards the achievment of "Goal 12: Responsible consumption and production."
BLOG
Please feel free to contact us for new product development or OEM consultation.
Development Partners
The cooperation of many people supports our manufacturing.
(Titles omitted, in no particular order)
Jichi Medical University
University of Tsukuba
National Cancer Center Hospital East
MOTEKI FOODS ENGINEERING CO., LTD.